45 fda structured product labels
SPL Xforms | FDA To register, please submit the following information via e-mail to spl@fda.hhs.gov: Attendee's first and last name. Name of your organization. E-mail address. Session name and date of training ... Structured Product Labeling (SPL) | Data Conversion Laboratory Structured Product Labeling (SPL) is a standard used by the FDA community to facilitate the communication of drug labeling data reliability among various groups such as the FDA, hospitals, prescribing organizations, doctors, and the general public. SPL is an HL7 and ANSI approved standard.
Structured Product Labeling Resources - fda.gov The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. SPL...
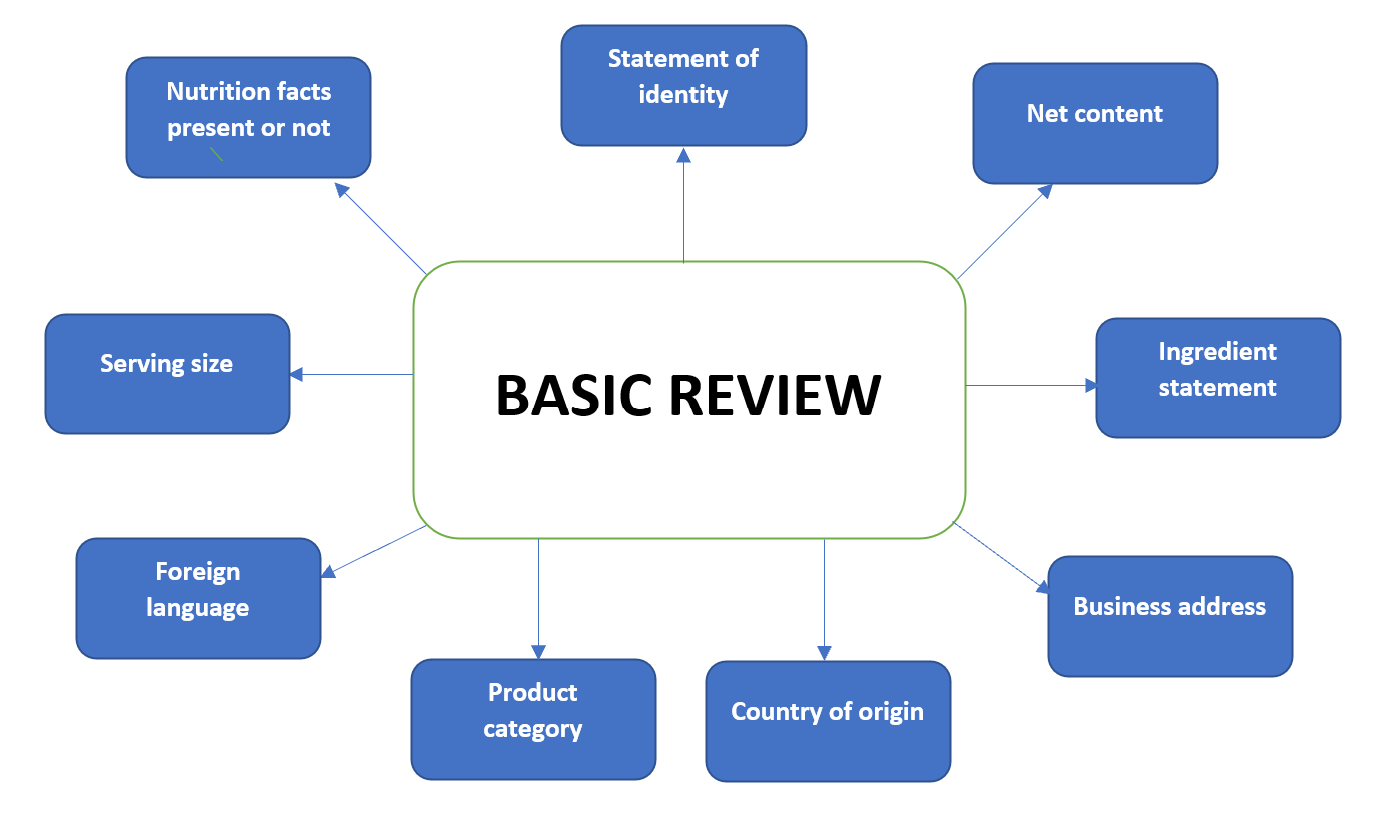
Fda structured product labels
Structured Product Labeling Resources | FDA The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. SPL... Nsde | Fda CMS Memo - PDE Editing using the FDA Online Label Repository (PDF) With the exception of the billing unit data in the NSDE document, this file is generated from SPL documents sent to FDA for... Structured Product Labeling Validation Rules Guidance for Industry - Indexing Structured Product Labeling (Final) Guidance for Industry: Self-Identification of Generic Drug Facilities, Sites, and Organizations ... 4 Drug Labeling, Listing ...
Fda structured product labels. › standard-operating-proceduresStandard Operating Procedures Templates | Smartsheet May 14, 2018 · Use this SOP template in Word to detail approved vendors and processes for ordering material, catalog where shipments are received, document how deliveries should be verified before acceptance, and keep track of how paperwork (such as packing slips and labels) is processed. Download Warehouse Standard Operating Procedure Template - Word › drugs › laws-acts-and-rulesPrescription Drug Labeling Resources | FDA Feb 24, 2022 · Improving the Accuracy of Structured Product Labeling (SPL) Submissions – The Missing LOINC (2019 presentation and video) Electronic Drug Registration and Listing Instructions › media › 154586Food and Drug Administration The FDA-483 document with indicated PRODUCT AREA of DRUGS for: Aetna Felt Corporation 2401 W. Emaus Ave., Allentown, PA, dated: 5/13/2021-5/13/2021 ... structured dataset of when drug applications ... FDA Label Search The device labeling has been reformatted to make it easier to read but its content has not been altered nor verified by FDA. The device labeling on this website may not be the labeling on currently...
Document Type including Content of Labeling Type | FDA loinc code. loinc name. 98075-5: animal cells, tissues, and cell and tissue based product label: 77647-6: animal compounded drug label: 86445-4: blanket no changes certification of product listing Indexing Structured Product Labeling | FDA Center for Drug Evaluation and Research Center for Biologics Evaluation and Research This guidance explains that FDA's Center for Drug Evaluation and Research (CDER) and Center for Biologics... FDA Infant Formula Update: June 10, 2022 | FDA The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines ... Structured Product Labeling Validation Rules Guidance for Industry - Indexing Structured Product Labeling (Final) Guidance for Industry: Self-Identification of Generic Drug Facilities, Sites, and Organizations ... 4 Drug Labeling, Listing ...
Nsde | Fda CMS Memo - PDE Editing using the FDA Online Label Repository (PDF) With the exception of the billing unit data in the NSDE document, this file is generated from SPL documents sent to FDA for... Structured Product Labeling Resources | FDA The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. SPL...

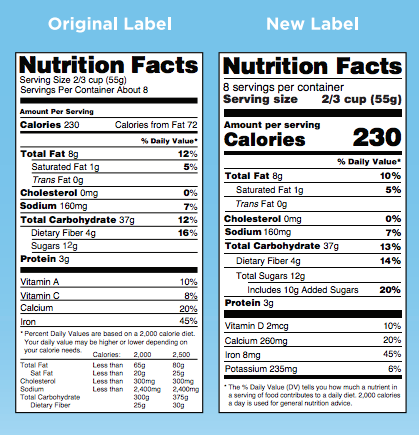








Post a Comment for "45 fda structured product labels"