42 structure function claims on dietary supplement labels
FDA Labeling Guidance: Making Structure/Function Claims ... The FDA permits supplements to so-called "structure-function claims," which are claims that describe the role of a nutrient or dietary supplement "intended to affect the structure or ... CH 2 - NUTRITION Flashcards - Quizlet CH 2 - NUTRITION. Dietary Reference Intakes (DRIs) are the recommended amounts of nutrients strictly to prevent deficiency. Structure/function claims on dietary supplement labels are not regulated as rigorously as health claims. Nice work!
Federal Preemption of 'Structure/Function' Claims on ... By definition, a structure/function claim "describes the role of a nutrient or dietary ingredient intended to affect the structure or function in humans [or] characterizes the documented mechanism...

Structure function claims on dietary supplement labels
Structure Function Claims - Dietary Supplement Experts Structure/function claims describe the role of a nutrient or dietary ingredient intended to affect the structure or function of humans, or characterize the documented mechanism (s) of action by which a nutrient or dietary ingredient acts to maintain such structure or function. Importantly, they cannot be disease claims. Is My Patient Taking an Unsafe Dietary Supplement ... To date, the FDA has found over 1060 tainted products marketed as dietary supplements. Dietary supplement claims. Three types of claims can be made about dietary supplements: health, structure/function, and nutrient content claims. GUIDANCE: Federal Labeling Requirements for Herbal Dietary ... The table and descriptions below provide an overview of the primary required information on dietary supplement labels and the panel on which the information should appear. Principal Display Panel Information Panel (panel to the immediate right of PDP) Any label panel • Statement of Identity (e.g., herbal supplement. or . dietary supplement) – MUST include the …
Structure function claims on dietary supplement labels. STRUCTURE/FUNCTION CLAIMS ON DIETARY SUPPLEMENTS - USFDA Blog The manufacturers or labellers often describes the role of a product or an ingredient characteristic to affect normal structure or functions in humans either by statement or pictures on dietary supplements.They are called as structure/function claims if and only if it is not linking to a disease in the context. NUTRITION SPOTLIGHT D Flashcards | Quizlet helps maintain a healthy heart Overall, the regulations around structure/function claims on dietary supplements on labels are: -informative and useful in guiding consumers to making decisions about a product's benefits. -meant to protect customers but are often misleading and overstate a product's benefits. Dietary Supplements Claims, Labels and Regulations | NSF Structure/function claims refer to the supplement's effect on the body's structure or function, including its overall effect on a person's well-being. Examples of structure/function claims include "Calcium builds strong bones" and "Antioxidants maintain cell integrity". Nutrient Content Claims Dietary Supplements - Claims and Labeling Compliance for ... Dietary supplements can make 'structure/function' claims (for example, 'calcium builds strong bones'). A structure/function claim describes the product's role in maintaining the 'structure or function of the body,' or 'general well-being.' Labeling rules are detailed. Depending on the ingredients, different rules come into play.
Up & Up & Out. Structure/Function Claims Preempted by the ... As a general matter, structure/function claims "describe the role of a nutrient or dietary ingredient intended to affect the structure or function in humans or that characterizes the documented mechanism by which a nutrient or dietary ingredient acts to maintain such structure or function[.]" 21 C.F.R. § 101.93(f). Constructing structure and function claims | Natural ... Assuming a product bears a proper structure/function claim and not a disease claim, supplement firms making these claims on their labels must still meet several requirements. While FDA does not preapprove structure/function claims, marketers must submit a notification to FDA that includes the text of the claim no later than 30 days after first ... Structure/Function Claims in Dietary Supplement Labeling ... Structure/Function Claims in Dietary Supplement Labeling: Not All of These Claims Need to Be Submitted to FDA and Accompanied in Labeling by the DSHEA Disclaimer Stephen H. McNamara * I. Introduction One important means of promoting dietary supplements is the inclusion, in label Glucosamine - Wikipedia Dietary supplement. Oral glucosamine is a dietary supplement and is not a prescription drug. Glucosamine is marketed as a supplement to support the structure and function of joints, and the marketing is targeted to people suffering from osteoarthritis.. Commonly sold forms of glucosamine are glucosamine sulfate, glucosamine chondroitin, glucosamine …
Label Claims for Food & Dietary Supplements | FDA Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,... nutr chapter 2 Flashcards - Quizlet If a nutrient is listed as "an excellent source" on a label, it contains at least 20% DV for that nutrient T/F Structure/function claims on dietary supplement labels are not regulated as rigorously as health claims. Nutraceutical - Wikipedia Depending on its ingredients and the claims with which it is ... may claim to prevent chronic diseases, improve health, delay the aging process, increase life expectancy, or support the structure or function of the body. Dietary supplements. Dietary supplements, such as the vitamin B supplement shown above, are typically sold in pill form. In the United States, the … Structure/Function Claims Basics - Dietary Supplement Experts Structure/function claims describe the role of a nutrient or dietary ingredient intended to affect the structure or function of humans, or characterize the documented mechanism (s) of action by which a nutrient or dietary ingredient acts to maintain such structure or function. Importantly, they cannot be disease claims.
Label Claims for Conventional Foods and Dietary ... Health claims describe a relationship between a food substance (a food, food component, or dietary supplement ingredient), and reduced risk of a disease or health-related condition. There are three ways in which FDA exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary su...
Solved Question 21 Structure/function claims on a dietary ... Transcribed image text: Question 21 Structure/function claims on a dietary supplement O must acknowledge that the FDA supports the claims that are misleading can lead to the product being taken off the market prove that the supplement can prevent a disease prove that the supplement can treat a disease O have to be approved, as done for drugs, before they can appear on product labels
Which of the following statements about structure/function ... A) Structure/function claims are illegal. B) Products with structure/function claims that are misleading cannot be taken off the market. C) Structure/function claims prove supplements can prevent or cure diseases. D) Structure/function claims do not have to be approved before they can appear on product labels. advanced-nutrition
Structure/Function Claim Notification for Dietary Supplements Office of Dietary Supplement Programs (HFS-810) Center for Food Safety and Applied Nutrition Food and Drug Administration 5001 Campus Drive College Park, MD 20740-3835 Contact the Office of Dietary...
Dietary Supplement Labeling Guide: Chapter VI. Claims | FDA you must use a disclosure statement when you make a nutrient content claim and your food (including dietary supplements) contains one or more of the following nutrients in excess of the levels...
Federal Preemption of 'Structure/Function' Claims on ... By definition, a structure/function claim "describes the role of a nutrient or dietary ingredient intended to affect the structure or function in humans [or] characterizes the documented mechanism by which a nutrient or dietary ingredient acts to maintain such structure or function." Id. at §343 (r) (6).
Structure/Function Claims | FDA Mar 07, 2022 · The Dietary Supplement Health and Education Act of 1994 (DSHEA) established some special regulatory requirements and procedures for structure/function claims and two related types of dietary...
Dietary Supplement Label Claims: What's Allowed? What's ... A structure/function claim is a statement that describes the role of a nutrient or dietary ingredient intended to affect the structure or function in humans or that characterize the documented mechanism by which a nutrient or dietary ingredient acts to maintain such structure or function, provided that such statements are not disease claims.



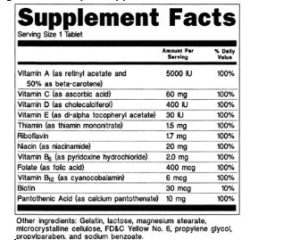

Post a Comment for "42 structure function claims on dietary supplement labels"